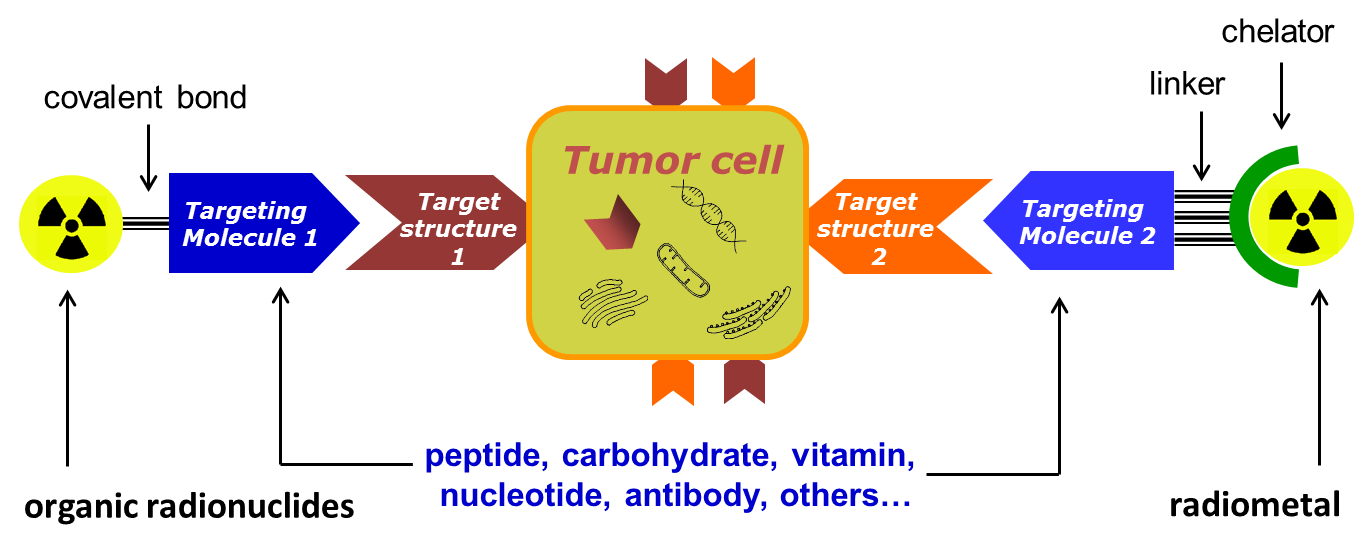
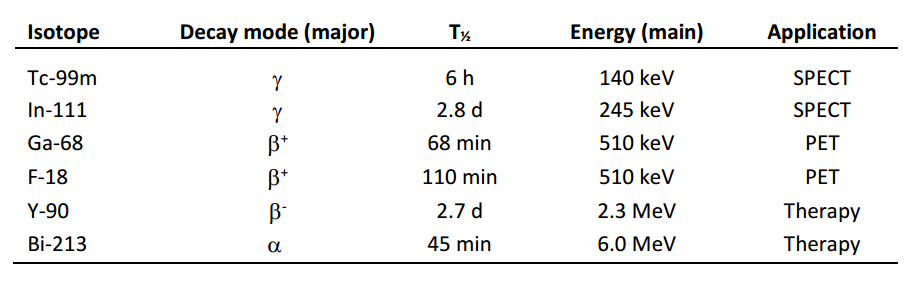
Radiopharmaceuticals of interest are conjugates that combine a radionuclide specific for a given application (Table 1) with a biological vector that is specific for a recognition element overexpressed by tumour cells (Figure 1). Depending on the application, the nature of the radionuclide and the vector molecule, different chemical and biological techniques are required for their preparation.
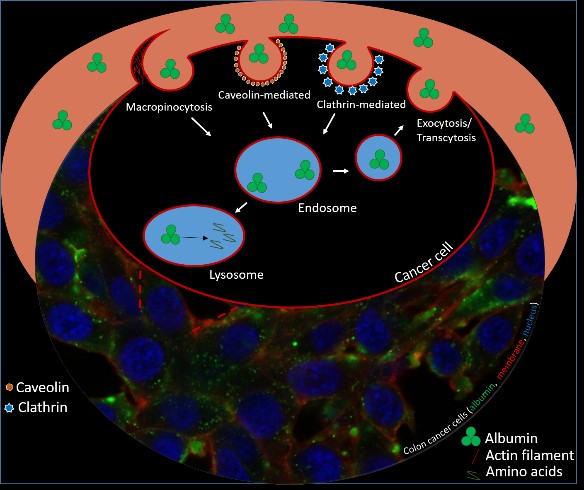
For preclinical evaluation, the radiopharmaceuticals are first tested in vitro (on cells) for their tumour-targeting properties, which usually includes experiments determining cellular uptake, affinity towards a given receptor/antigen, and other parameters (e.g., metabolic and enzymatic stability, hydrophilicity on the basis of physicochemical methods and new applied models, plasma protein binding, cell internalisation.). Promising candidates are then carried forwards to in vivo investigations (in mice) for determination of their pharmacokinetic and -dynamic properties employing biodistribution experiments and/or small animal imaging by µSPECT/PET/CT.